Enhancing Recruitment, Linkage to Care and Treatment for HIV-Infected Men Who Have Sex with Men (MSM) in the United States
What was HPTN 078?
HPTN 078 was a US-based research study designed to develop and determine the effectiveness of a combined HIV prevention strategy that included a method to identify, recruit, and link men who have sex with men (MSM) and transgender women (TGW) to HIV care and an intervention to help people living with HIV achieve and maintain viral suppression (low level of HIV in the body).
Who participated in the study?
Overall 1,305 people (95% MSM, 4% TGW) were recruited in four US cities (Atlanta, Ga.; Baltimore, Md.; Birmingham, Ala.; and Boston, Mass.). Of the 1,305, 144 people living with HIV who were not virally suppressed were enrolled into the study and followed for 12 months.
Why is HPTN 078 important?
According to the U.S. Centers for Disease Control and Prevention, MSM continue to be the group most heavily affected by HIV in the US. While MSM represent approximately two percent of the US population, they accounted for nearly 67% of newly diagnosed HIV infections in the US in 2014. There is an urgent need to develop innovative methods to identify MSM who are HIV-infected and not virally suppressed, as well as ways to help MSM remain in care and achieve viral suppression. Achieving viral suppression is critical both for individual health and to stop HIV transmission within this community.
What happened during the study?
HPTN 078 was the first HPTN study to use a recruitment method known as deep-chain respondent driven sampling (DC-RDS) to identify and recruit HIV-infected MSM. DC-RDS uses a small group of participants, known as “seeds”, who are well connected within the population to recruit other MSM they know into the study. The MSM recruited by the “seeds” repeat the process and refer other MSM they know and so on. The study team assessed the ability of DC-RDS to identify and recruit HIV-infected MSM in the US who are not virally suppressed.
The enrolled MSM were randomized to either the standard of care (SOC) or the study intervention arm, which was designed to enhance engagement in HIV care. Participants randomized to the intervention arm worked with a case manager (CM), who helped the participant link to and remain engaged in HIV care, including health services navigation and ART adherence counseling. Participants in the intervention arm also had the option to receive automated reminders for ART adherence and appointments via text, email and phone. The control arm used the SOC for linkage to care, ART adherence and retention in care. The study team compared how well the intervention and SOC arms achieved viral suppression 12 months after enrollment.
Results
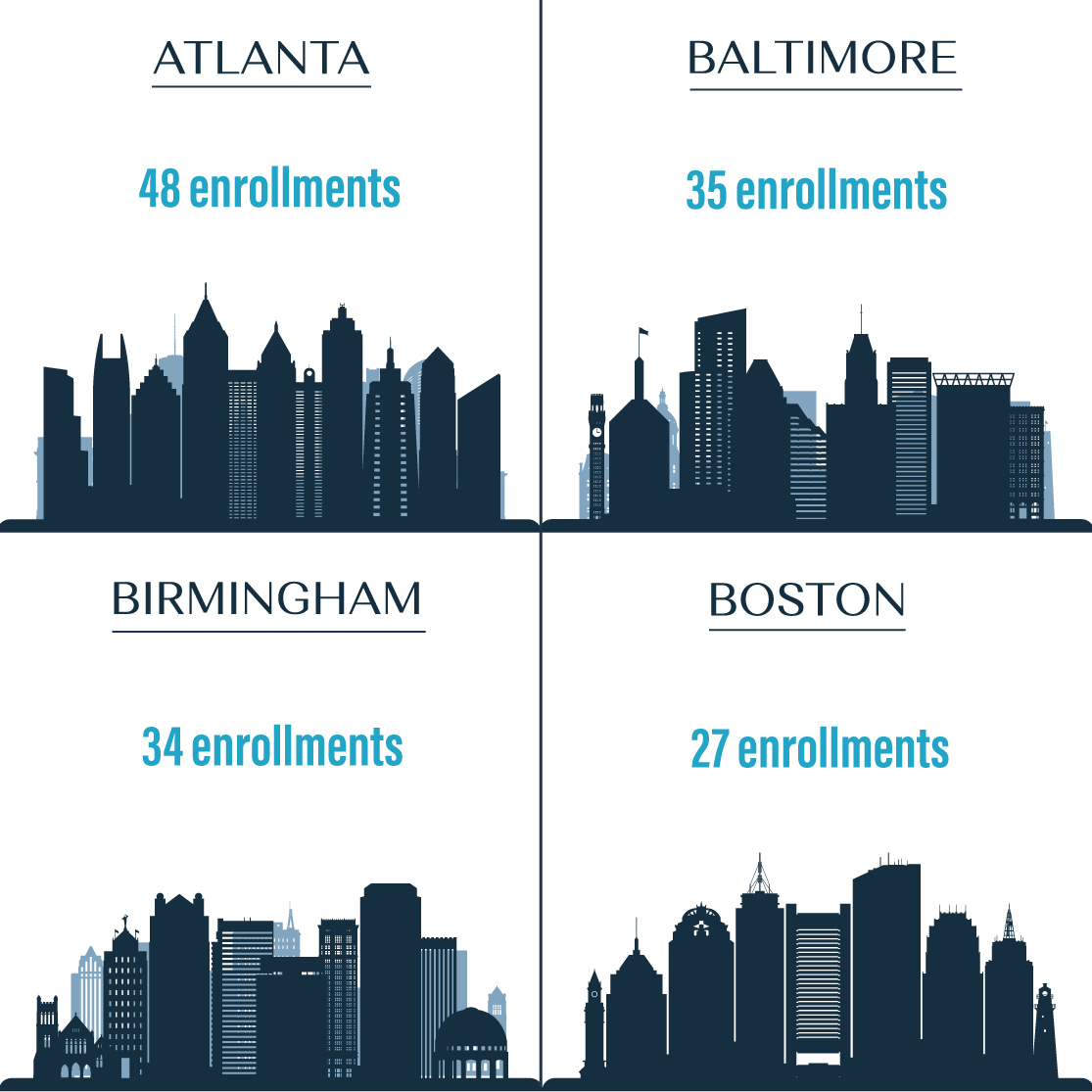
Of the 1,305 MSM and TGW screened at four U.S. sites, 144 study participants were enrolled.
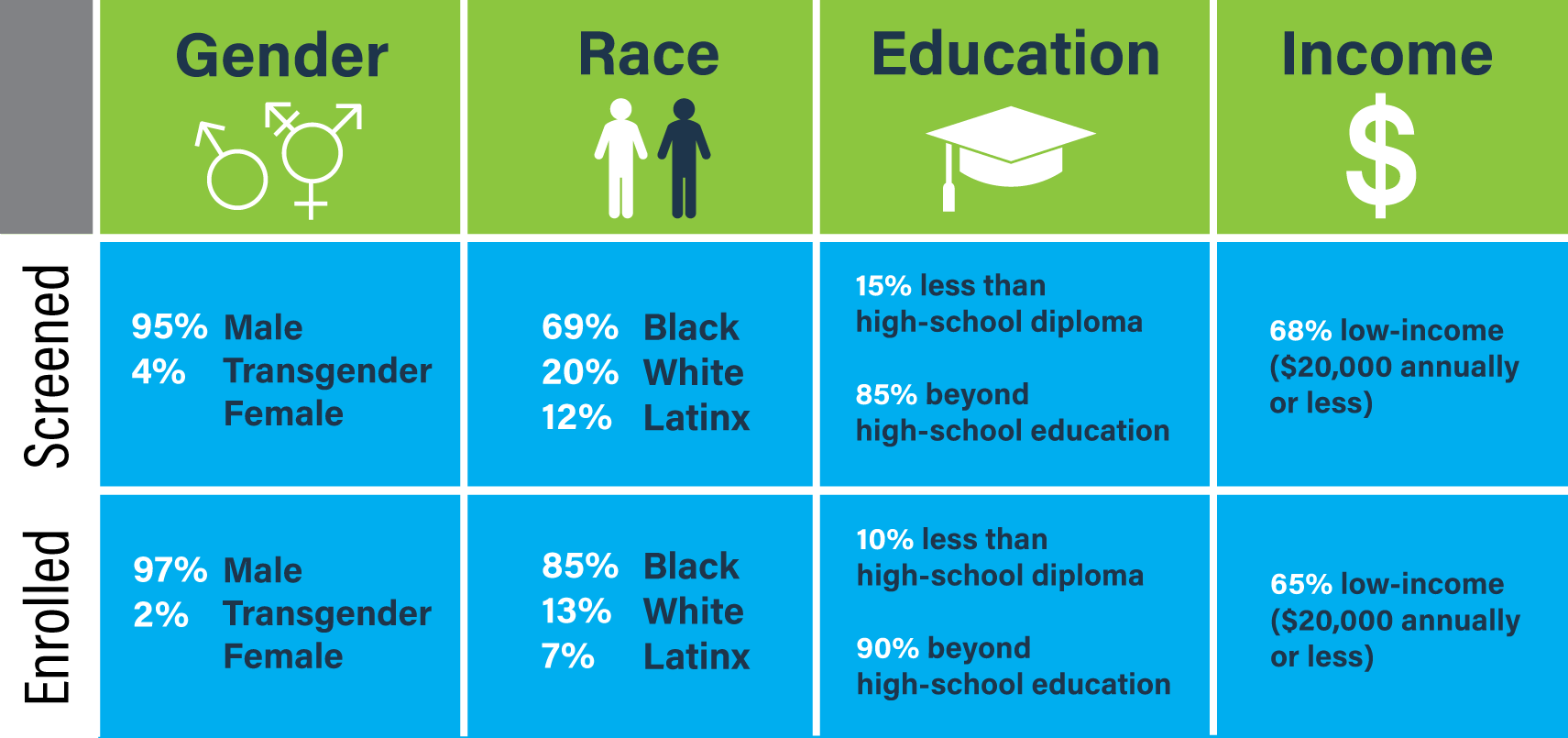
Nearly all participants who were screened (95%) and enrolled (97%) identified as male. A majority were African American (69% screened, 85% enrolled), had education beyond high-school (85% screened, 90% enrolled), and had an annual income of $20,000 or less (68% screened, 65% enrolled).
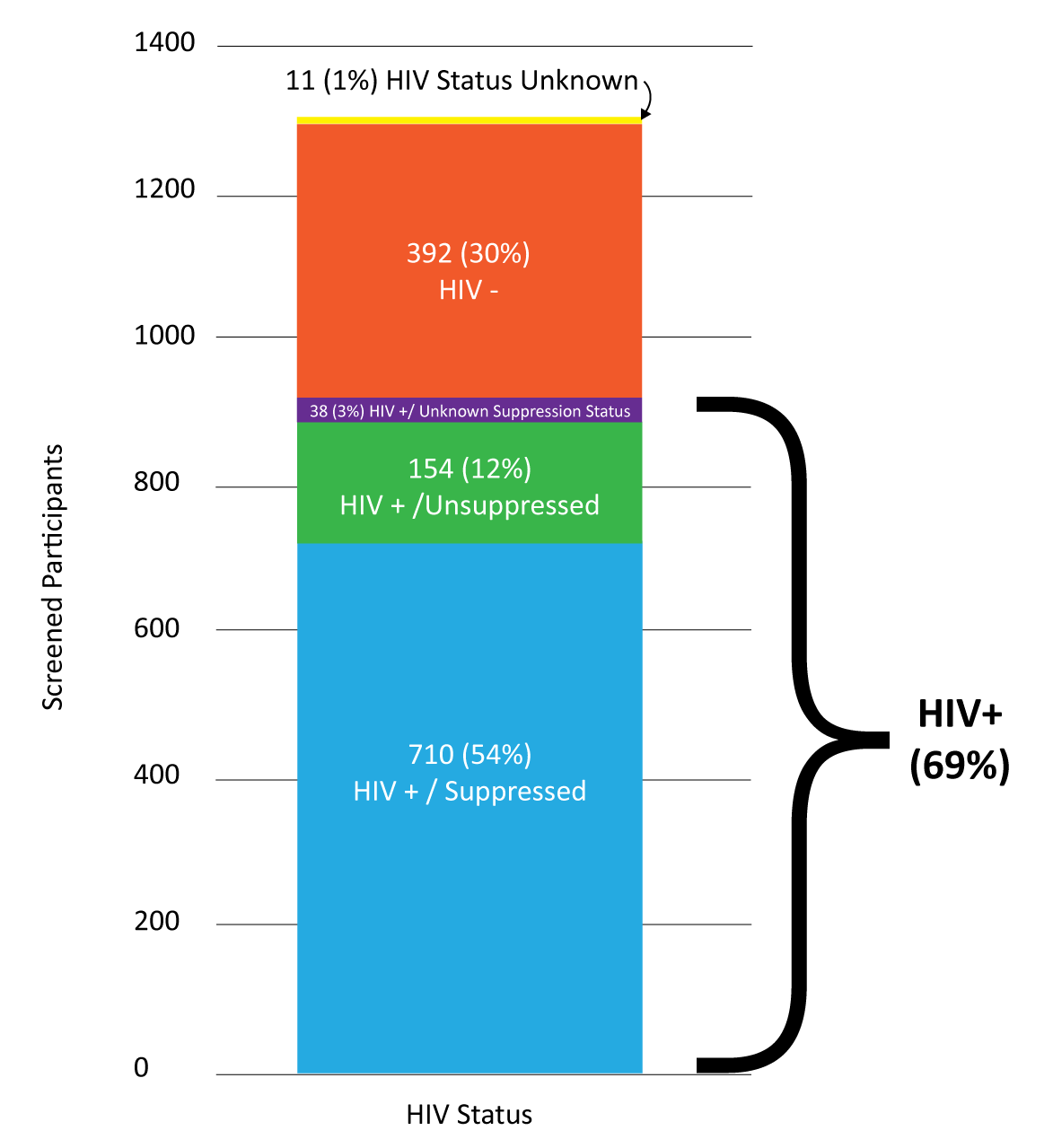
Overall, 902 of the 1305 screened participants were living with HIV. 144 (94%) of the eligible 154 people living with HIV who were not virally suppressed were enrolled into the study. Screened participants living with HIV who were virally suppressed (710 people) were not eligible for HPTN 078.
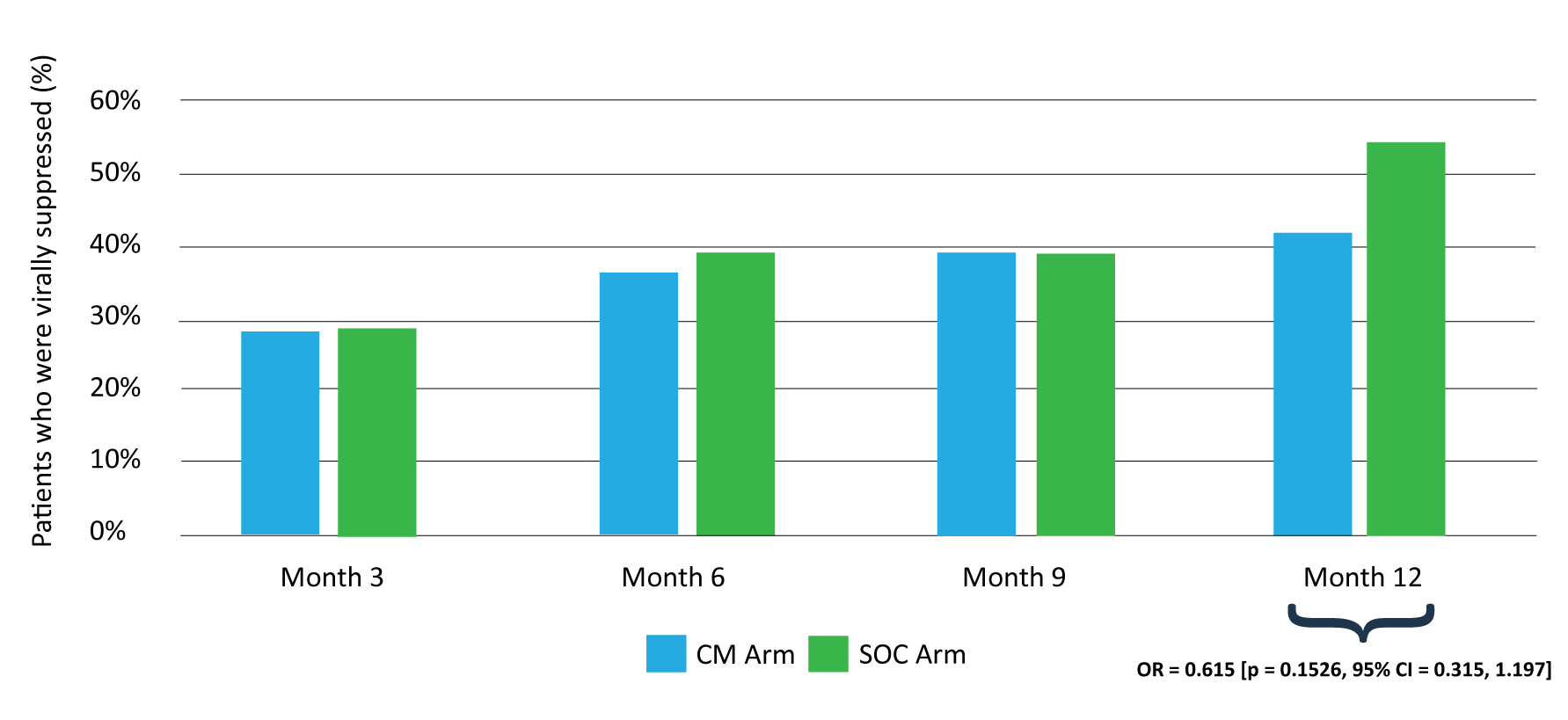
After one year, 91 percent of the participants remained in follow-up in the study with 48 percent achieving viral suppression. However, there was no difference in viral suppression noted between participants who received the case management intervention and those who received the standard of care.
There was an increase in overall viral suppression over time – 28 percent at 3 months, 36 percent at 6 months, 39 percent at 9 months.
Study Documents
HPTN 078 Version 2.0
HPTN 078 Version 1.0
- HPTN 078 Protocol V1.0 - 8 Oct 2015
- LoA #5 - 19 April 2017
- LoA #4 - 20 December 2016
- LoA #3 - 23 June 2016
- LoA #2 - 5 May 2016
- LoA #1 - 15 Jan 2016
Presentations
- HIVR4P 2016: Mathematical Modelling for HPTN 078
- HIVR4P 2018: Understanding HIV Transmission Dynamics and the Impact of Past HIV Interventions among MSM in Baltimore: a Modelling Study for HPTN 078
- HIVR4P 2018: Finding Men Who Have Sex With Men (MSM) Who May Be Potential Amplified HIV Transmitters: Results From HPTN 078
- HIVR4P 2018: Differences in Engagement in HIV Prevention, Treatment, and Care by Wave of Respondent-Driven Sampling Recruitment among Gay men and other Men who have Sex with Men in Four US Cities: Results from HPTN 078
- CROI 2019: HPTN 078: High Prevalence of HCV Antibodies Among Men Who Have Sex With Men (MSM)
- CROI 2019: Contribution of HIV Disease and Care Stages to HIV Transmission Among Baltimore MSM: A Modelling Study for HPTN 078
More Study Documents
You must request access to private documents (i.e. SSPs) in Microsoft Teams. You can request access to the Team by emailing Jeff Webb (jwebb@fhi360.org).
You will need a Microsoft account to log in to Team. Click here for instructions on setting up your Microsoft account and accessing the Teams platform.
Study Details
The purpose of this study is to develop and assess the efficacy of an integrated strategy that includes feasible and scalable interventions to identify, recruit, link to care, retain in care, attain, and maintain viral suppression among HIV-infected men who have sex with men (MSM) in the United States (US).
This study will use deep-chain respondent driven sampling (DC-RDS) and direct recruitment to identify and recruit HIV-infected MSM who are not virally suppressed. A subset of these men will be enrolled into one of two study arms. The intervention arm will provide a Case Manager (CM) intervention package designed to enhance linkage to care, antiretroviral treatment (ART) initiation, treatment adherence and retention in care. The control arm will provide the standard of care (SOC) for linkage to care, initiation of ART, treatment adherence and retention in care. The primary outcome of the study is viral suppression 12 months after enrollment. Phylogenetic methods will be used to evaluate the relationship between social and sexual networks identified through DC-RDS and viral networks. Mathematical modeling will be performed using demographic, behavioral, and clinical data generated from this study and other sources to estimate the population-level impact of the intervention on HIV incidence and to estimate the level of identification, linkage, ART coverage and viral suppression that would be required to achieve a substantial reduction in HIV incidence among MSM in the US settings where the study is conducted.
HIV-infected and HIV-uninfected adolescent (> 16 years old) and adult MSM in selected US cities will be screened for this study. Enrollment will be limited to HIV infected men who are not virally suppressed.
Approximately 2700 MSM will be identified and recruited using both a DC-RDS strategy and direct recruitment (DR) in four cities; 356 HIV-infected MSM who are not virally suppressed will be randomized (1:1) to the intervention (enhance case management) and control study arms of the study.
The overall study duration is 48 months: 24 months for DC-RDS recruitment and enrollment; 12 months of follow-up for participants who are randomized into the intervention and control study arms; and approximately 12 months after the completion of participant study visits for data analyses, phylogenetic assessments and modeling.
There are no specific drug regimens under investigation in this study.
• Assess the ability of DC-RDS to identify and recruit HIV-infected MSM in the US who are not virally suppressed.
• Compare the efficacy of the two study arms (intervention vs. SOC) in achieving durable viral suppression (defined as HIV VL < 200 copies/ml) 12 months after enrollment.
• Assess HIV prevalence and the proportion of HIV-infected men who are virally suppressed by comparing early wave (approximately 1-6) vs. later (deep) wave (approximately 7-12) DC RDS participants.
• Compare the proportion of men in the two study arms who are virally suppressed at 3, 6, 9, and 12 months after enrollment.
• Assess linkage to care and retention in care in the two study arms by comparing the 1) proportion of men with at least one care visit within 30 days of enrollment, 2) time to the first care visit, and 3) proportion of men with at least four care visits (one in each six month interval, with at least 60 days between these visits) over the 12 months after enrollment.
• Compare the proportion of men with HIV-hepatitis C virus (HCV) co-infection in the two study arms who are linked to care (defined as one care visit within 30 days of enrollment) and who achieve (HIV) viral suppression 12 months after enrollment.
• Examine the association between baseline behavioral, socio-demographic, and clinical characteristics (including syphilis) of HIV-infected men and viral suppression status for all men screened via DC RDS and for the men in the two study arms 12 months after enrollment.
• Compare the changes in sexual risk behavior, health care utilization, stigma, substance use and mental health between the two study arms over 12 months by administering questionnaires at baseline and 12 months after enrollment.
• Evaluate the feasibility and scalability of the intervention package by measuring the number of intervention contacts (e.g., text message, email, phone, in person) per participant over 12 months.
• Compare the experience of linkage to and ongoing HIV care among participants in the two study arms by conducting and analyzing exit interviews.

