Integrating HIV Prevention, Gender-Affirmative Medical Care, and Peer Health Navigation to Prevent HIV Acquisition and HIV Transmission for Transgender Women in the Americas: A Vanguard Feasibility and Acceptability Study

What is HPTN 091?
HPTN 091 is a study assessing the feasibility, acceptability, preliminary impact of a multi-component strategy that provides HIV prevention services, gender-affirming hormone therapy, and Peer Health Navigation to improve pre-exposure prophylaxis (PrEP) uptake and adherence.
Who is participating in HPTN 091?
This study will be enrolling about 310 transgender women (TGW), ages 18 or older, not living with HIV.
Why is HPTN 091 important?
Transgender women bear a disproportionate burden of HIV infection globally. Gender-affirming hormone therapy is an unmet need and community priority for TGW. An accepted and feasible intervention that delivers HIV prevention services with hormonal therapy could significantly impact the HIV epidemic among TGW.
What will happen during HPTN 091?
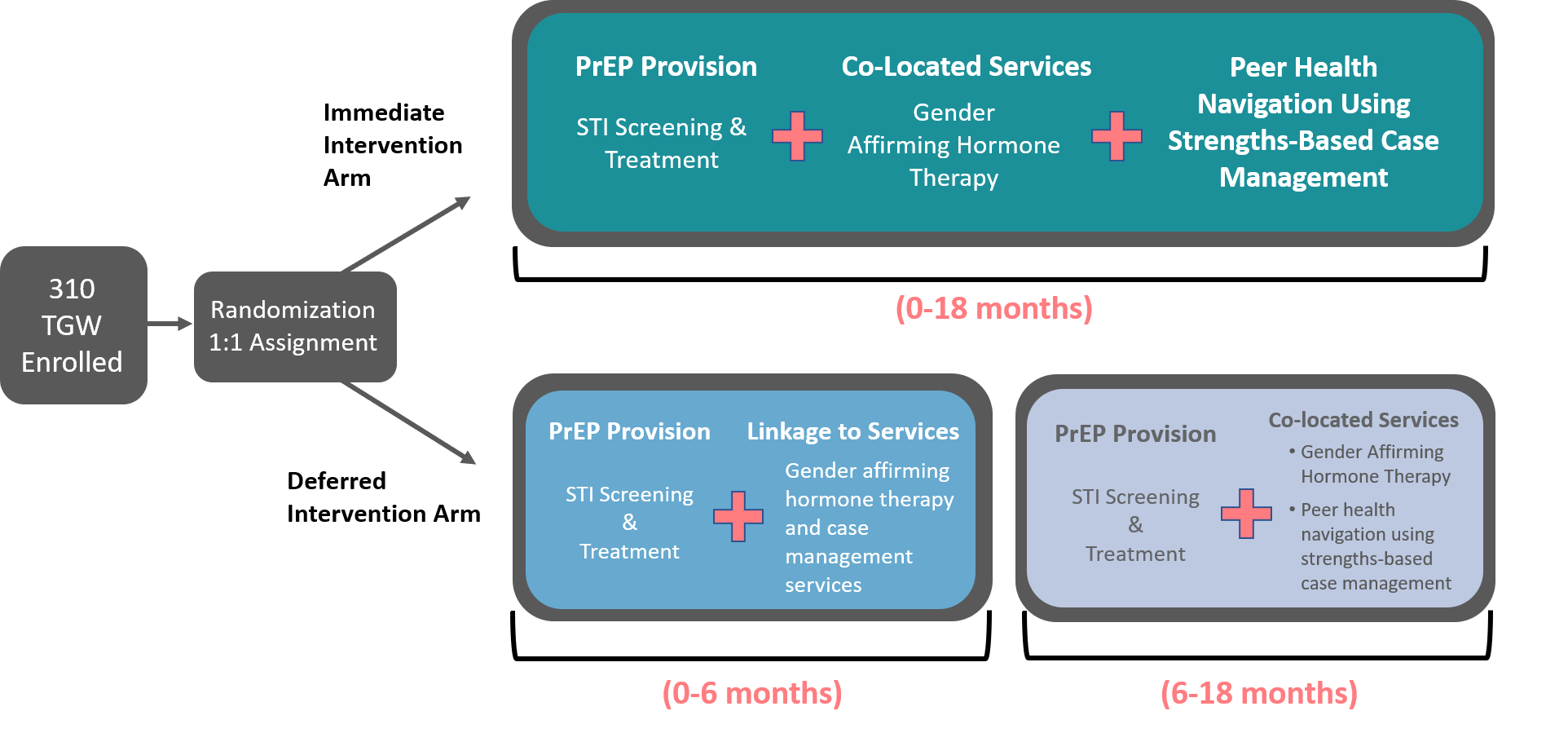
Participants will be randomized to two arms: an Immediate Intervention Arm and a Deferred Intervention Arm.
- Immediate Intervention Arm: participants will receive PrEP provision, co-located services and Peer Health Navigation using Strengths-Based Case Management for 18 months.
- Deferred Intervention Arm: for the first 6 months, participants will receive PrEP provision and linkage to services. From 6-18 months, participants in this arm will receive the full intervention of PrEP provision, co-located services and Peer Health Navigation using Strengths-Based Case Management.
Study Documents
HPTN 091 Version 1.0
- HPTN 091 V1.0 - 13 April 2020
- HPTN 091 V1.0 LoA #1 - 26 May 2021
- HPTN 091 V1.0 LoA #2 - 22 July 2021
- HPTN 091 V1.0 LoA #3 - 05 Oct 2022
HPTN 091 SSP
- Section 01 - Introduction V1.2 - 20 Oct 2022
- Section 02 - Document Requirements V1.2 - 20 Oct 2022
- Section 03 - Accrual Retention V1.1 - 20 Oct 2022
- Section 04 - Study Procedures V1.3 - 18 Apr 2023
- Section 05 - Study Product Considerations V1.1 - 20 Oct 2022
- Section 06 - Clinical Considerations V1.2 - 20 Oct 2022
- Section 07 - AE Reporting V1.0 - 28 Jan 2021
- Section 08 - Lab Specimen Management Procedures V1.3 - 18 Apr 2023
- Section 09 - Counseling and Behavioral Considerations V1.0 - 28 Jan 2021
- Section 10 - Data Management V1.2 - 20 Oct 2022
- Section 11 - Data Communiques V1.1 - 10 Aug 2021
- Section 12 - Study Reporting Plan V1.1 - 10 Aug 2021
- Appendix I - IDI V1.2 - 20 Oct 2022
- Appendix II - GAHT Considerations V1.2 - 20 Oct 2022
- Appendix III - PHN V1.1 - 10 Aug 2021
- SSP Version Control V1.3 - 18 Apr 2023
More Study Documents
You must request access to private documents (i.e. SSPs) in Microsoft Teams. You can request access to the Team by emailing Jeff Webb (jwebb@fhi360.org).
You will need a Microsoft account to log in to Team. Click here for instructions on setting up your Microsoft account and accessing the Teams platform.
Study Details
To assess the feasibility, acceptability, and preliminary impact of a multi-component strategy to improve pre-exposure prophylaxis (PrEP) uptake and adherence that integrates delivery of biomedical HIV prevention co-located with gender-affirming transgender care (hormonal therapy and medical monitoring) and Peer Health Navigation (PHN) using Strengths-Based Case Management (SBCM) professional supervision.
Multi-site, open-label study with each participant randomized 1:1 to Immediate Intervention vs. 6-month Deferred Intervention Arms. Both arms will be provided PrEP and sexually transmitted infection (STI) screening and treatment. Participants in the Immediate Intervention Arm will receive co-located gender-affirming medical care and PHN using SBCM starting at the Enrollment Visit. Participants in the Deferred Intervention Arm will receive linkage to external gender-affirming medical care and case management services during the deferred period and will transition to the study intervention six months following the Enrollment Visit.
Transgender Women (TGW), ages 18 or older, HIV-uninfected, at risk of acquiring HIV infection.
Approximately 310 HIV-uninfected TGW.
For each individual site, the duration of the study is approximately 36 months from the time of site activation. Accrual will require approximately 15 months (3 months for Implementation testing and 12 months for general study); individual participants will be followed for 18 months. Once enrolled, each participant will complete eight follow-up visits.
The Intervention will include an approved PrEP agent (Descovy® and Truvada® in the U.S., Truvada® in Brazil), STI screening and treatment, co-located gender-affirming medical care, and PHN using SBCM.
• To assess acceptability and feasibility of delivering integrated HIV prevention services co-located with gender-affirming hormone therapy (GAHT) and PHN using SBCM for TGW.
• To assess PrEP uptake, adherence, and persistence in both the Immediate Intervention Arm and the Deferred Intervention Arm and compare uptake, adherence and persistence of PrEP between the two arms.
• To determine annual incidence of HIV infection among study participants.
• To determine baseline prevalence and annual incidence of STIs (Neisseria gonorrhoeae [GC], Chlamydia trachomatis [CT], and Treponema pallidum (syphilis)) and to examine changes in STI incidence over time by study arm.
• To examine changes in sexual risk-taking behavior (e.g., number of serodiscordant or HIV-unknown serostatus sexual partners; number of condomless anal/vaginal sex episodes with these partners).
• To obtain baseline laboratory data to evaluate the cohort’s suitability for future PrEP intervention studies (e.g., prevalence of renal insufficiency, hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, etc.).
• To identify demographic, behavioral, socioeconomic, and psychosocial factors related to: (1) PrEP uptake and PrEP adherence over time, (2) PrEP persistence, and (3) interest in future HIV research, including research involving injectable and implantable agents for PrEP among HIV-uninfected TGW.
• To assess use of medically-prescribed and non-prescription gender-affirming interventions, including exogenous hormones, soft tissue fillers/silicone, and feminizing surgeries (e.g., breast augmentation, vaginoplasty/labiaplasty, orchiectomy, etc.).
• To explore beliefs about drug interactions between gender-affirming hormonal therapy and antiretroviral (ARV) drugs used for PrEP.
• To evaluate TGW experiences of participating in an interventional study that includes biomedical and behavioral assessments, including the social impact of participation.
• To assess the safety of the intervention.
• To use laboratory assessments to characterize incident HIV infections. These assessments may include viral load testing, HIV drug resistance testing, ARV drug testing, phylogenetic analysis, and analysis of the host response to HIV infection.
• To examine the potential impact of hormonal therapy on PrEP concentrations.

