
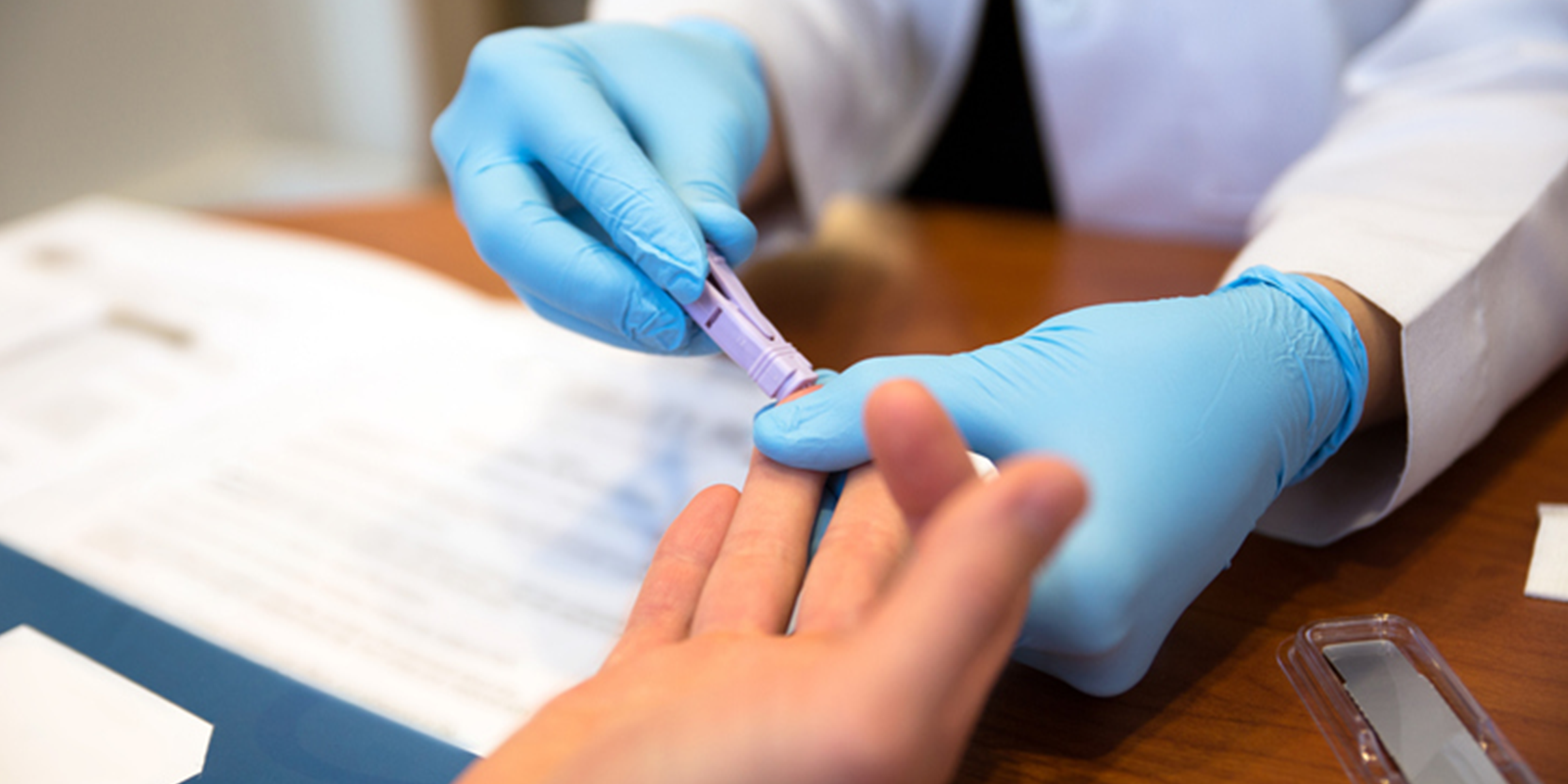
HPTN research studies are developed by protocol teams that include CTU investigators and recognized experts in HIV prevention. As study protocols are developed, they undergo a rigorous intra- and extra-network review process that ensures compliance with current ethical guidelines and regulatory procedures.
Each Study Protocol is available once version 1.0 is approved and distributed to the performance sites. The CORE Protocol Contact can provide additional information and materials.