HPTN 076
Phase II Safety and Acceptability of an Investigational Injectable Product, TMC278 LA, for Pre Exposure Prophylaxis (PrEP)

- Study Summary
- Details
- Documents
- Personnel
- Sites
- Publications
What is HPTN 076?
HPTN 076 is a Phase II clinical research study that was designed to find out if a new form of the drug rilpivirine is safe and acceptable for use as HIV Pre-Exposure Prophylaxis (PrEP). The pill form of rilpivirine is U.S. FDA-approved and is used for treatment of HIV. HPTN 076 studied the new, long-acting liquid form of rilpivirine which stays in the body for months and is given by injection into the buttock muscle.
Who participated in the study?
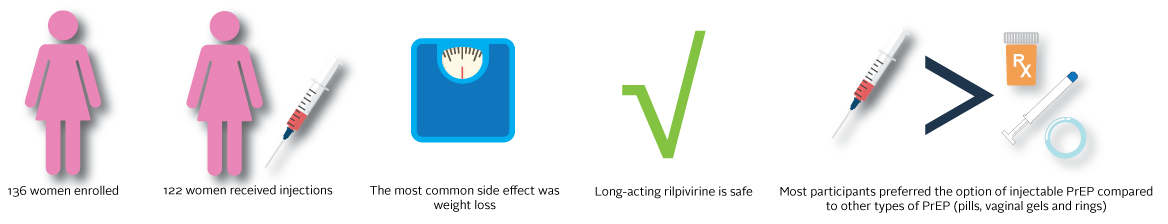
HPTN 076 enrolled a total of 136 HIV-uninfected women in four cities: Cape Town, South Africa; Harare, Zimbabwe; Newark, New Jersey; and Bronx, New York. Study participants were at low risk for becoming HIV-infected, were between the ages 18-45 years old, and able to provide informed consent.
Why is HPTN 076 important?
HPTN 076 was an important next step to determine if TMC278 LA could be used as injectable PrEP. This study is one of the first to see if TMC278 LA is safe and acceptable when used by HIV-uninfected women. The results of HPTN 076 may help researchers decide whether TMC278 LA should be tested further to see if new HIV infections are prevented by the drug. Future studies may help researchers understand whether people are more adherent to injectable PrEP (TMC278 LA) than to daily oral PrEP (Truvada®).
What happened during the study?
HPTN 076 study started in March 2015 and ended in March 2017. At the beginning of the study, participants were randomly placed into one of two groups. One group of women received the new, long-acting rilpivirine; the other group received no active drug (they received a placebo). The placebo looked like the long-acting rilpivirine so neither the participants nor study staff knew which group was receiving the new rilpivirine drug.
Before participants could receive an injection, they had medical evaluations to make sure they were healthy. Next, all participants took a pill daily for four weeks (either a rilpivirine pill or a placebo pill which looked like the rilpivirine pill). Pills were given for the safety of the participants. The drug in the pill passes through the body in about a day; however, the drug in the long-acting injection remains in the body for several months. It is not possible to remove the long-acting rilpivirine from a participant once it is injected. By giving participants pills first, the research team could make sure no one had negative side effects before giving an injection of the long-acting rilpivirine.
Participants who had no serious side effects after taking pills began study injections. Injections were given every eight weeks for 40 weeks at six different clinic visits. Throughout this time, participants received injections, medical information was collected and safety lab tests were done to make sure participants were not having negative side effects. Also, during the time period participants were receiving injections they were asked questions about what they liked and/or disliked about the long-acting injectable. After the last injection visit, face-to-face group discussions with a subset of study participants were held to help researchers better understand participant feelings about the long-acting rilpivirine as an option for PrEP.
Results
Safety Results
Long-acting rilpivirine is safe. Overall there was no difference in the number and type of side effects (adverse events) between the study group receiving long-acting rilpivirine and the study group receiving placebo.
- 67 of the 80 (84%) women who received long-acting rilpivirine injections had side effects.
- 33 of the 42 (79%) women who received placebo injections had side effects.
- The most common side effect was weight loss.
Acceptability Results
Women liked the option of a long-acting injectable form of PrEP.
- Most participants preferred the option of injectable PrEP compared to other types of PrEP (pills,
vaginal gels and rings). - About 80% of the participants liked that the injectable is easier to use than some other HIV
prevention methods. - About 70% of the participants liked that the injectable can offer longer-term HIV protection.
- More than 75% of the participants rated the number, size, location, and frequency of the
injections as very acceptable. - Most participants (61-67%) reported pain with the injection but found the pain to be acceptable.
Protocol Status
Concluded
Study Purpose
To evaluate the safety and acceptability of the injectable product, TMC278 LA, in healthy, human immunodeficiency virus (HIV)-uninfected women.
Study Design
This is a multi-site, double-blinded, two-arm, two:one, randomized, trial comparing the safety of an intramuscular (IM) injection of TMC278 LA to a placebo given once every eight weeks over a 40 week period among sexually active, HIV-uninfected women. Approximately 132 women will be randomized.
Study Population
HIV-uninfected women, ages 18-45.
Study Size
The study will include approximately 132 women (defined as those receiving at least one injection) randomized as follows: 88 in the active product arm and 44 in the placebo arm.
Study Duration
Total study duration is estimated to be approximately 100 weeks. Accrual will require approximately 24 weeks. Each subject will be enrolled and followed for a total of 76 weeks. Study participants will receive oral product for four weeks, then IM injections at eight week intervals (Weeks 4, 12, 20, 28, 36 and 44), and will be followed for 32 more weeks.
Treatment Regime
Participants will be randomized to either the active product or the placebo arm. Participants randomized to active product will first be prescribed oral TMC278 (rilpivirine) 25 mg capsule, once daily for four weeks as an oral run-in and then will receive IM injections of active TMC278 LA, 1200 mg (formulation G001) at eight week intervals (Weeks 4, 12, 20, 28, 36 and 44). On each dosing occasion, 1200 mg of TMC278 LA will be delivered in two, 2 mL injections, one in each gluteus maximus muscle. Participants randomized to placebo will first be prescribed daily oral placebo for four weeks and then will receive saline (0.9% NaCl) in two, 2 mL injections, one in each gluteus maximus muscle at eight week intervals (Weeks 4, 12, 20, 28, 36 and 44).
Primary Objectives
To evaluate the safety of the injectable product, TMC278 LA (1200 mg dose administered at Weeks 4, 12, 20, 28, 36 and 44), through 48 weeks after initial injection (at Week 52) in women in sub-Saharan Africa (SSA) and the US.
Secondary Objectives
- To evaluate the tolerability and acceptability of TMC278 LA.
- To describe the pharmacokinetics (PK) of TMC278 LA in plasma.
- To describe TMC278 LA concentration in cervicovaginal fluid and rectal fluid in participants, and in cervicovaginal tissue in a subset of approximately 24 participants (US sites only).
- To evaluate the safety of rilpivirine (oral + injectable LA product) through Week 76 in women in SSA and the US.
- To estimate HIV incidence through study follow-up.
Other Objectives
Study Documents
HPTN 076 Version 3.0
HPTN 076 Version 2.0
- HPTN 076 Protocol V2.0 – 01 Oct 2014
- Version 2.0: Letter of Amendment 2 – 13 Mar 2015
- Version 2.0: Letter of Amendment 1 – 03 Mar 2015
- Version 2.0: Clarification Memo #1 – 19 Nov 2015
HPTN 076 Version 1.0

Linda-Gail Bekker
Protocol Chair

Zvavahera Michael Chirenje
Protocol Team Member

Lynda Marie Emel
SDMC Project Manager

Jennifer Farrior
CORE Protocol Specialist

Craig Hendrix
Protocol Team Member

Jessica Justman
Protocol Team Member

Sue Li
Protocol Team Member

Alex London
Protocol Team Member

Estelle Piwowar-Manning
Protocol Team Member

Huguette Redinger
Protocol Team Member

Paul Richardson
Protocol Team Member

Katie Shin
DAIDS Pharmacist

Nirupama Sista
Protocol Chair

Jill Stanton
CORE Protocol Specialist

Betsy Tolley
Protocol Team Member

Rhonda White
Protocol Team Member

Peter Williams
Protocol Team Member