Population Effects of Antiretroviral Therapy to Reduce HIV Transmission (PopART): A cluster-randomized trial of the impact of a combination prevention package on population-level HIV incidence in Zambia and South Africa
HPTN 071 Public Use Datasets are now available:
https://dataverse.harvard.edu/dataverse/HPTN-071

Study Documents - Study Details - Key Study Personnel - Study Sites - Publications
What is HPTN 071?
HPTN 071 - Population Effects of Antiretroviral Therapy to Reduce HIV Transmission (PopART) examined the impact of a package of HIV prevention interventions on community-level HIV incidence. The prevention interventions included universal voluntary HIV counseling and testing provided at the household level, linkage of HIV infected individuals to care and early initiation of antiretroviral therapy (ART) for all those testing HIV-positive. The study was conducted in 21 communities in the Western Cape of South Africa, and in Zambia.
Who participated in the study?
Twenty-one (21) urban and peri-urban communities in Zambia and South Africa, with a total population of around 1 million, participated in this study. To measure the impact of the intervention, a research cohort called the Population Cohort, consisting of a sample of approximately 2,300 adults aged 18–44 years, were recruited from the general population in each of the 21 communities (an overall total of 48,302 across all communities) and was followed up once a year for three years to measure HIV incidence and other outcomes.
Why was the study conducted?
HIV incidence rates remain at very high levels in many parts of southern Africa. More effective prevention strategies are needed to steeply reduce the number of new HIV infections and to ensure that care can be provided for all people living with HIV. Findings from HPTN 071 (PopART) will help inform the scale-up of future HIV programs and identify cost-effective interventions.
What was the design of the study?
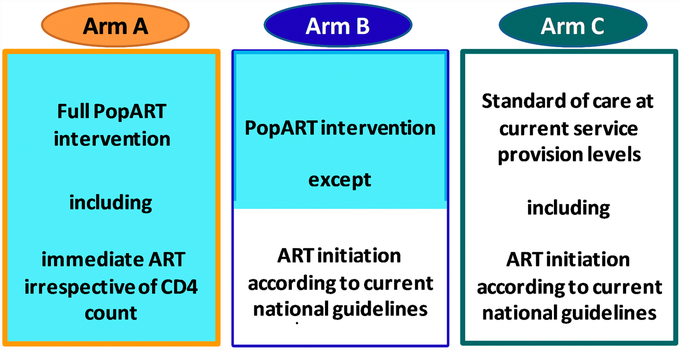
A total of 21 communities participated in the HPTN 071 (PopART) trial, 12 in Zambia and nine in South Africa. In Zambia, the 12 communities were divided into four matched “triplets” of three communities each. The triplets were composed of communities that had been matched so all three had similar estimated HIV prevalence, to minimize the variability in baseline HIV incidence within the triplet. Then the communities in each triplet were randomly assigned to the three study arms: A, B and C. The same process was repeated for the nine communities in South Africa. In both countries, Arm A received the full PopART HIV combination prevention package including the offer of universal ART, Arm B received the PopART package, but with ART only offered to those eligible according to in-country guidelines, and Arm C communities had no household intervention but could access HIV testing and treatment services according to in-country guidelines.
What were the study objectives?
The primary study outcome was HIV incidence between month 12 and month 36 among members of the Population Cohort who were HIV-negative at baseline, comparing HIV incidence between the intervention and standard of care arms, (A vs C, and B vs C). This allowed the effectiveness of the PopART intervention at population level to be measured. Data to investigate secondary objectives were drawn from Population Cohort data as well as data from Community HIV-care Providers (CHiPs), health centers and social science research. These objectives assess the effect of the intervention on several additional variables, including:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What was included in the PopART intervention in Arms A and B?
The PopART HIV combination prevention package in Arms A and B was delivered throughout each study community by Community HIV-care Providers (CHiPs). They were specially trained, lay counselors, who worked in pairs. Each pair was responsible for delivering services to a zone consisting of around 500 households. CHiPs were tasked with visiting every household at least once a year for the duration of the trial, and provided the following services:
- Household-based HIV counseling and testing offered annually
- Linking those with HIV to care at the local health center
- Follow-up visits to clients living with HIV to ensure they had linked to care and to support ART adherence
- Promoting voluntary medical male circumcision for men who tested HIV-negative
- Promoting services for the prevention of mother-to-child transmission (PMTCT) to pregnant women living with HIV
- Referral to treatment of sexually transmitted infections
- Providing condoms in the community
- Screening and referral for tuberculosis (TB)
How did the study design evolve over time?
HIV care and ART were provided at the local government clinic serving that community. In Arm A, all clients living with HIV were offered ART irrespective of CD4 count or clinical stage. In Arm B, ART was initiated according to in-country guidelines. Initially, this was at a CD4 count of 350, then later this changed to 500, and in 2016, following a change in World Health Organization (WHO) recommendations, ART was offered to all clients living with HIV, as in Arm A.
The dates shown for the start of universal ART (i.e., ART irrespective of a patient's CD4 cell count or WHO stage) refer to when this was implemented in study clinics in the respective countries. In Zambia, the first study clinic transitioned April 19, 2016, and the last May 9, 2016. This transition is represented by the dark purple band in the figure. In South Africa, the first study clinics transitioned October 10, 2016, and the last on November 21, 2016, a period represented by the dark purple band in the figure. Study personnel, clinic staff and implementing partners worked to ensure study clinics implemented the new policy as soon as in-country guidelines and logistics such as staff training would allow. Transition to universal ART in study clinics, therefore, often preceded transition in neighboring communities.
What were the key findings from the HPTN 071 (PopART) study?
Findings from HPTN 071 (PopART) show delivery of an HIV prevention strategy that includes offering HIV testing to everyone, with immediate referral to HIV care, and treatment for people living with HIV based on prevailing in-country guidelines, can substantially reduce new HIV infections. In the study arm where the PopART intervention included HIV treatment according to in-country guidelines (Arm B), HIV incidence declined by 30 percent compared to standard of care communities (Arm C), an improvement that was highly statistically significant. In both intervention arms (A and B), the first two of the UNAIDS 90-90-90 targets (regarding knowledge of HIV status and being on treatment) were reached and high rates of viral suppression were achieved. In the study arm where the PopART intervention included the offer of universal ART even before it was national policy (Arm A), incidence only declined by seven percent compared to the standard-of-care arm, and this was not statistically significant. The lack of a clear effect in Arm A was surprising, and not explained by observed rates of viral load suppression. Further analyses underway may help explain this finding. These results, clarified by further analysis and in conjunction with those from other universal test and treat trials, will help guide policymakers on how best to leverage community-based HIV testing and linkage to care to achieve HIV control. Separately, projections from mathematical modelling and cost-effectiveness analyses were presented at the 10th International AIDS Society Conference on HIV Science in Mexico City (IAS 2019). Researchers found a continuation of community-wide HIV testing and prompt initiation of treatment as delivered in the study could lead to substantial reductions in new HIV cases, be cost-effective, and help to achieve the UNAIDS 2030 targets.
How did HPTN 071 (PopART) differ from the other UTT trials?
HPTN071 (PopART) differed from the other trials in the following ways:
1. The PopART communities were large urban or peri-urban communities whereas the other trials were in smaller rural communities.
2. HIV incidence was measured in a separately recruited population cohort in PopART and Ya Tsie/BCPP, while in TasP and SEARCH incidence was measured through the intervention.
3. In PopART and Ya Tsie/BCPP, the control communities did not receive any additional intervention outside of current standard of care, while SEARCH and TasP control communities received enhanced community-wide HIV services, particularly for HIV testing and linkage to care, probably explaining the lack of observed difference between the intervention and control arms.
What additional HPTN 071 (PopART) study analyses are underway?
HPTN 071 (PopART) researchers are currently examining the effects of the interventions on other study outcomes including herpes simplex virus-2 incidence, tuberculosis and HIV-related stigma.
Study Documents
- FAQ
- HPTN 071 Brochure
- HPTN 071 Individual-based Model Analysis Plan
- HPTN 071 Cost Effectiveness Analysis Plan
- HPTN 071 Statistical Analysis Plan
HPTN 071 Version 3.0
- HPTN 071 Protocol V3.0 - 16 Nov. 2015
- HPTN 071 Summary of Changes from V2.0 to V3.0 - 16 November 2015
- HPTN 071 V3.0 LoA #5 - 17 April 2018
- HPTN 071 V3.0 LoA #4 - 27 Sept. 2017
- HPTN 071 V3.0 LoA #3 - 6 June 2017
- HPTN 071 V3.0 LoA #2 - 6 Dec. 2016
- HPTN 071 V3.0 LoA #1 - 23 Aug. 2016
HPTN 071 Version 2.0
HPTN 071 Version 1.0
- HPTN 071 Protocol V1.0 - 26 Oct. 2012
- HPTN 071 V1.0 CM #2 - 17 October 2013
- HPTN 071 V1.0 CM #1 - 21 August 2013
Ancillary and Sub-Studies
- HPTN 071 Phylogenetics Ancillary Protocol V2.0 - 6 July 2017
- HPTN 071 Phylogenetics Ancillary Protocol V1.0 - 15 January 2015
- HPTN 071 Stigma Substudy V1.0 - 6 January 2014
Presentations
- Understanding and Unpacking the Results of the HPTN 071 (PopART) Trial: HIV R4P 2021 (Video)
- IAS 2019 Symposia Session: Towards Zero New HIV Infections: Lessons Learnt From the HPTN 071 (PopART) Trial
- HPTN 071 (PopART) Trial: Impact of a UTT Intervention on HIV Incidence (Dr. Richard Hayes and Dr. Sarah Fidler)
- Model Projections of the Impact of the PopART Intervention (Dr. William Probert)
- Cost and Societal Value of the Household-Based Combination HIV Prevention Intervention Implemented in HPTN 071 (PopART) (Dr. Katharina Hauck)
- Community and Stakeholder Engagement in the HPTN 071 (PopART) Trial (Dr. Musonda Simwinga)
- HPTN 071 Key Outcomes Web Conference - 27 March 2019
- HPTN 071 at AIDS 2018
- HPTN 071 at IAS 2017
- HPTN 071 Satellite Session at IAS 2017 ("Cross-Disciplinary Approaches to Understand Successes and Challenges of Implementing a Community-Wide Universal Test and Treat Programme in Sub-Saharan Africa")
- HPTN 071 at CROI 2017
- HPTN 071 at AIDS 2016
- HPTN 071 at CROI 2016
- HPTN 071 - Key Findings As Study Nears Completion
- HPTN 071 - Key Findings After the Third Year of Intervention
- HPTN 071 - Key Findings After the Second Year of Intervention
- HPTN 071 - Key Findings After One Year of Intervention
Press
- July 2019: Intensive Anti-HIV Efforts Meeting with Mixed Success in Africa (New York Times)
- July 2019: HPTN 071 Modelling and Economic Analyses Show Benefits of Community-wide HIV Testing and Treatment
- March 2019: HPTN 071 Demonstrates Community-Wide HIV Prevention Strategy Can Reduce New Infections
- July 2018: HPTN Responds to Results from SEARCH and BCPP Studies Presented at AIDS 2018
- July 2016: HPTN Responds to Results from ANRS 12249 TasP Study Presented at AIDS 2016
- May 2016: Immediate ART Available to All People Living With HIV in HPTN 071 (PopART) Zambia Communities
Web Conference
More Study Documents
You must request access to private documents (i.e. SSPs) in Microsoft Teams. You can request access to the Team by emailing Jeff Webb (jwebb@fhi360.org).
You will need a Microsoft account to log in to Team. Click here for instructions on setting up your Microsoft account and accessing the Teams platform.
Study Details
The purpose of this study is to determine the impact of two community-level combination prevention packages, both of which include universal HIV testing and intensified provision of HIV antiretroviral therapy (ART) and care, on population-level HIV incidence.
This is a three-arm, cluster-randomized, longitudinal study to be implemented in 21 clusters (communities).
The prevention packages will be implemented throughout the communities randomized to the intervention arms. Main study outcomes will be measured in a randomly-selected group drawn from the adult population of the communities: a Population Cohort.
The combined population of all 21 clusters is approximately 1 million individuals. The interventions will be implemented in 14 of the 21 clusters with a combined population of approximately 645,000 individuals (adults and children) in the intervention arms. The approximate sizes of the randomly-selected groups for main study outcome assessments are:
1) Population Cohort: 42,000 individuals; 2) Case-Control Studies: 1,600 individuals; 3) Qualitative Studies: about 2,000 individuals; 4) Population Cross-Sectional Survey: 10,500 individuals (if funded)
The planned duration of the entire study will be approximately 6 years, with delivery of the intervention, and enrollment and follow-up of the Population Cohort, occurring over 4 years. Assessment of the primary outcome (HIV incidence) in the Population Cohort is planned to take place 12, 24, and 36 months after recruitment.
Arms A and B* - Full PopART Intervention delivered by Community HIV-care Providers (CHiPs) including:
a) Offering voluntary HIV counseling and testing annually through a house-to-house campaign
b) Linking those with HIV to care at the local health center
c) Offering ART to all those who are HIV-infeced, irrespective of CD4+ count or clinical stage
d) Promoting voluntary medical male circumcision (VMMC) for men who test HIV-negative
e) Promoting services for the prevention of mother-to-child transmision (PMTCT) to HIV-infected pregnant women
f) Referral for treatment of sexually transmitted infections
g) Providing condoms in the community
h) Screening and referral for tuberculosis (TB)
Arm C* - Standard of Care (Control Arm)
a) Strengthening of standard HIV testing and ART services according to local guidelines* at health facilities and other venues
b) Strengthening of male circumcision and PMTCT services available at health facilities and other venues in the community
c) Strengthening of STIs treatment and provision of condoms at health facilities and other venues in the community
d) ART to all those who are HIV-infeced, irrespective of CD4+ count or clinical stage*
*In the original design of the study, Arm A communities received the full PopART intervention wherase Arm B communities received the PopART intervention except with ART initiation according to local guidelines (based on CD4 count or clinical stage). In late 2015, in response to mounting evidence of clinical benefit, the World Health Organization guidelines on HIV/AIDS were revised to recommend ART for all people living with HIV. The study team responded by incorporating this recommendation into the study design, making ART available for all people living with HIV in all study arms.
To measure the impact of the two intervention packages on HIV incidence by enrolling and following a random sample of adults (the Population Cohort) in the trial communities for 3 years.
Measure the impact of the two intervention packages on the following: 1) HIV incidence over each year of follow-up; 2) Community viral load (subject to funding); 3) ART adherence and viral suppression (subject to funding); 4) ART drug resistance (subject to funding); 5) HSV-2 incidence; 6) Uptake of HIV testing and retesting over the entire study period; 7) ART screening and uptake; 8) Time between HIV diagnosis and initiation of care; 9) Retention in care; 10) HIV disease progression and death; 11) ART toxicity; 12) Sexual risk behavior; 13) Case notification rate of TB; 14) HIV-related stigma; 15) Uptake of PMTCT; and 16) Uptake of male circumcision.
Carry out case-control studies to examine factors related to: 1) Uptake of HIV testing during the first round of home-based testing in Arms A and B; and 2) Uptake of immediate treatment in Arm A
Use qualitative methods to: 1) Assess popular understanding of HIV testing and treatment at study initiation and during implementation; 2) Evaluate the acceptability and functioning of the CHiPs in Arms A and B; 3) Evaluate the acceptability of interventions and barriers to access in Arms A and B; 4) Document the effect of the interventions on social networks, stigma, sexual behavior, alcohol use, gender-based violence, HIV identity, other HIV prevention options and community morale; and 5) Evaluate the process and challenges of community consultation and applying ethical principles.
Measure the burden experienced by local health centers due to implementation of the intervention in the community.
Measure the incremental cost of the two intervention packages through systematic recording of costs in intervention and control communities
Estimate the effectiveness and cost-effectiveness of the intervention packages and alternative packages, both in the chosen study populations and in other populations by fitting mathematical models based on the empirical data from the trial, including data related to cost.